Post by : Mariam Al-Faris
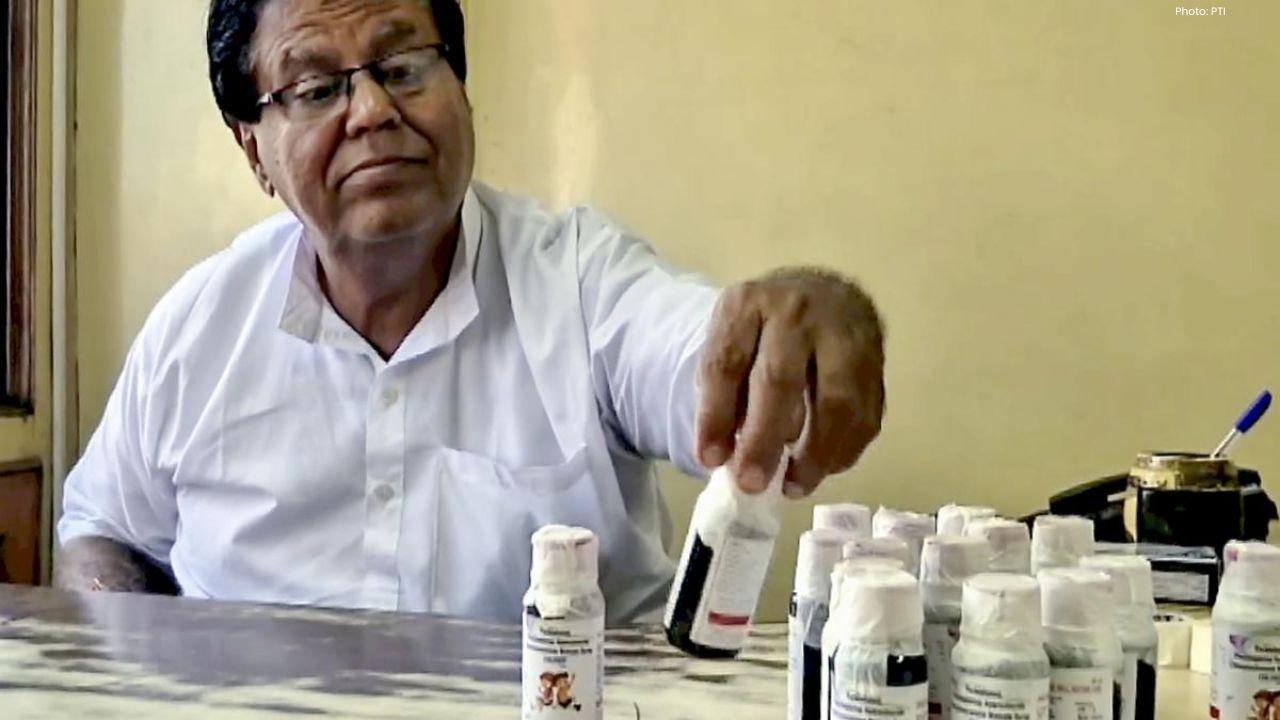
Ranganathan Govindan, the owner of Tamil Nadu-based Sresan Pharmaceuticals, has been arrested in connection with the adulterated Coldrif cough syrup that caused multiple children’s deaths across India. The Madhya Pradesh police took him into custody in Chennai late last night after extensive investigations into the scandal.
Deaths Linked to Poisonous Syrup
The case was filed after at least 20 children in Madhya Pradesh and several in Rajasthan died following the consumption of Coldrif syrup. The children suffered kidney infections caused by toxic substances in the cough syrup. Authorities charged Ranganathan with adulteration, culpable homicide not amounting to murder, and endangering the safety of children.
Meticulous Police Operation
Ranganathan had been on the run since the tragedy. Madhya Pradesh police, with the help of local Chennai authorities, launched a carefully planned midnight operation to arrest him. The team, which included drug inspectors, had been monitoring his movements and residence since October 5. He was caught around 1:30 am and taken to his company’s Kancheepuram factory, where crucial documents were seized.
Investigation to Widen
The police are now seeking a transit remand from a Chennai court to bring Ranganathan to Chhindwara, where most deaths occurred. Investigations will expand to cover the entire supply chain, including chemical suppliers, stockists, and medical representatives, to identify all parties responsible for distributing the toxic syrup.
Toxic Substance in Coldrif
Cold symptoms like runny nose, sneezing, sore throat, and watery eyes in children were treated using Coldrif. However, Tamil Nadu authorities found that the syrup contained diethylene glycol (DEG), a poisonous chemical used in inks and glue. DEG can severely damage kidneys, liver, and the nervous system. The factory was adding 46-48% DEG to the syrup, far exceeding the permitted limit of 0.1%.
Factory Violations and License Suspension
An inspection revealed unbilled containers of DEG in the Kancheepuram factory. Following these findings, the Tamil Nadu Drugs Control Authority issued a stop-production order, froze all stocks, and suspended the company’s license. Sresan Pharmaceuticals, originally registered in 1990, had continued operating even after being struck off the Ministry of Corporate Affairs register, raising concerns over regulatory oversight.
Widespread Impact and Bans
At least nine states have banned Coldrif syrup following the deaths. The case has exposed serious lapses in India’s pharmaceutical manufacturing and regulatory practices. The national drug regulator confirmed inspections showed not every batch of raw materials and active ingredients was being properly tested, highlighting systemic failures in ensuring medicine safety.








NBA Friday Highlights: Miami, Lakers, Milwaukee, and Clippers Triumph
Miami, Lakers, Bucks, and Clippers secure victories in thrilling NBA Friday games with standout perf

Doncic Dominates with 49 Points as Lakers Defeat Timberwolves 128-110
Luka Doncic scores 49 points to propel the Lakers past the Timberwolves 128-110; Reaves and Hachimur

Kings Narrowly Defeat Jazz 105-104 with Sabonis' Late Heroics
Domantas Sabonis' last-minute shot secures a thrilling 105-104 win for the Kings against the Jazz in

Argentina's Friendly Match with India Delayed, New Date to be Announced
Argentina's friendly against India in Kochi is postponed; a new date will be confirmed soon due to F

Rohit and Kohli Conclude ODI Journeys in Australia with a Win
Rohit Sharma and Virat Kohli close their ODI chapter in Australia with a win, partnering for an unbe

George Russell Dons Lucha Libre Mask at Mexican Grand Prix
George Russell sported a Lucha Libre mask to experience the Mexican GP stands while rookie Fred Vest